EZI-DOCK - Ezi-Flow™ CSV MK5 VHP
Improving Operator Safety and Optimising Powder Transfer Efficiency
The Ezi-Flow™ CSV MK5 VHP is a high-containment powder transfer system designed for pharmaceutical applications requiring sterility and operator protection. With validated Vaporised Hydrogen Peroxide (VHP) compatibility, it ensures enclosed, sterile transfer of APIs, high-potency compounds, and non-sterile powders.
Its closed-transfer design minimises cross-contamination risks and integrates seamlessly into isolators and cleanroom environments. By reducing the need for secondary containment and extensive PPE, it enhances operational efficiency while maintaining strict regulatory compliance. The CSV MK5 VHP system enables secure, repeatable material transfer, supporting contamination control in pharmaceutical manufacturing.




Sterile API handling | Enables contamination-free transfer of active pharmaceutical ingredients in aseptic environments. |
High-potency compound containment | Safely transfers HPAPIs and cytotoxic compounds while maintaining nanogram-level containment. |
Powder transfer in isolators and RABS | Ensures secure material transfer within restricted-access barrier systems and isolators without compromising sterility. |
Charging/discharging into process vessels | Facilitates controlled powder transfer into reactors, blenders, and formulation equipment while preventing exposure. |
Aseptic fill-finish operations | Supports final product formulation by maintaining sterile conditions during powder transfers. |
Bulk powder processing | Streamlines handling of excipients and intermediates in pharmaceutical production lines. |
- Full containmentProtects product and operator
- VHP compatibilityEnables effective decontamination
- Optimised powder flowReduces material loss
- Flexible integrationFits existing manufacturing setups
- Enhanced safetyMinimises PPE reliance
- Suitable for potent APIsEnsures regulatory compliance
- Containment Level: OEB 5 (nanogram-level containment)
- Material Compatibility: APIs, HPAPIs, cytotoxic compounds, excipients
- Transfer Capacity: Up to 100 kg per operation
- Sterilisation Method: Vaporised Hydrogen Peroxide (VHP) compatible
- Sealing Mechanism: Single-use, high-integrity containment seals
- Integration: Suitable for isolators, RABS, and cleanroom environments
- Regulatory Compliance: cGMP, FDA, and EU Annex 1 compliant
Traditional vs Advanced Powder Transfer in VHP Workflows
Traditional SBV systems from ChargePoint and Andocksysteme have long been the standard for powder transfer, but they often fall short in sterility, cleanability, and integration with VHP workflows.
The Ezi-Flow CSV MK5 VHP is specifically designed for reliable performance in VHP-decontaminated environments. It ensures effective sterilisation, seamless integration into isolators and RABS, and secure aseptic powder transfer. With high containment, optimised powder flow, and simplified handling, it’s an ideal solution for sterile pharmaceutical manufacturing.

Question or want to request a qoute?
Feel free to contact us.
Interested in improving your containment material transfer with Ezi-dock’s Ezi-FlowTM CSV System? Request a free, no-obligation quote, ask questions, or schedule a demo. Feel free to get in touch with us!
Phone: +31 85 043 3110
Email: info@romynox.nl

 Single-Use
Single-Use
 Containments
Containments Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Valves
Valves
 Seals
Seals
 Hoses
Hoses
 Fittings
Fittings
 Tube Sealers & Welders
Tube Sealers & Welders
 Transfer Ports
Transfer Ports
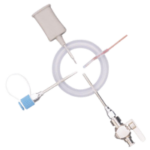 Validation
Validation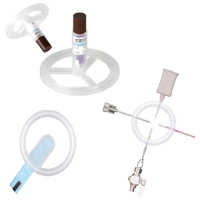


 We utilize cookies to ensure you have the best possible experience while visiting our website.
We utilize cookies to ensure you have the best possible experience while visiting our website.