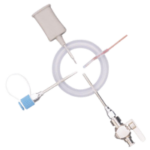
EZI-DOCK - ChargeBag with Tri-Clamp Connector
Secure and Efficient Powder Transfer for High-Containment Applications
Ezi-Dock Chargebags provide a high-integrity solution for secure, aseptic powder transfer in pharmaceutical and biopharmaceutical production. Designed for single-use applications, they reduce contamination risks while maintaining process control.
Constructed from high-quality, pharmaceutical-grade films, these chargebags support GMP and cGMP compliance and integrate seamlessly with cleanroom handling systems. Their robust design ensures safe, contamination-free transfer of potent materials, meeting stringent containment standards.


|
Aseptic Powder Transfer
|
Ideal for sterile manufacturing environments, ensuring contamination-free transfer of excipients, buffers, and active pharmaceutical ingredients (APIs).
|
|
High-Potency API (HPAPI) Handling
|
Designed for secure containment of highly potent or toxic compounds, protecting both operators and the environment.
|
|
Biopharmaceutical Production
|
Used in upstream and downstream processing to transfer raw materials, intermediates, and final products while maintaining sterility.
|
|
Sterile API Packaging
|
Enables safe storage and transfer of APIs and intermediates in controlled cleanroom conditions.
|
|
Closed-System Material Transfer
|
Minimises cross-contamination risks in cleanrooms, reducing the need for open handling of powders.
|
|
Customised Containment Solutions
|
Compatible with isolators, glove boxes, and other containment systems for highly controlled environments.
|
- High-Level Containment
Ensures secure, enclosed transfer of potent powders, protecting operators and products.
- Tri-Clamp Connection
Leak-proof, universal seal compatible with multiple process systems. - GMP & cGMP Compliance
Meets strict pharmaceutical production regulations.
- Aseptic Powder Handling
Reduces contamination risks for critical applications.
- Durable & Flexible
Supports high-integrity powder transfer with minimal material loss, reducing waste and simplifying handling.
- Material: Pharma-grade LDPE, 2-ply construction with anti-static properties
- Connection Type: Tri-Clamp, available in multiple sizes
- Containment Level: <1 µg/m³ (OEB 4 and 5 capable)
- Film Properties: Gamma-sterilisable, high-barrier, solvent-resistant
- Compliance: GMP, cGMP, USP Class VI, and FDA 21 CFR 177.1520
- Volume: Available in 1L to 50L configurations
Ezi-Dock Flexible Chargebags Brochure
Ezi-Dock PharmaPure AS Range Brochure
Comparison with Industry Chargebags
Compared to ChargePoint ChargeBag® and GEA Hicoflex®, Ezi-Dock Chargebags provide a more adaptable, user-friendly solution for powder transfer. The Tri-Clamp connection simplifies integration with existing process equipment, eliminating the need for proprietary connectors. This feature enhances flexibility and cost efficiency, making them a practical choice for high-containment material handling.

Question or want to request a qoute?
Feel free to contact us.
Interested in improving your powder containment and handling with Ezi-Dock Chargebags? Request a free, no-obligation quote, ask questions, or schedule a demo. Feel free to get in touch with us!
Phone: +31 85 043 3110
Email: info@romynox.nl

 Single-Use
Single-Use
 Containments
Containments Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Afsluiters
Afsluiters
 Afdichting
Afdichting
 Slangen
Slangen
 Fittingen
Fittingen
 Tube Sealers & Welders
Tube Sealers & Welders
 Transferports
Transferports
 Validatie
Validatie


 Wij gebruiken cookies om ervoor te zorgen dat u een optimale website-ervaring heeft.
Wij gebruiken cookies om ervoor te zorgen dat u een optimale website-ervaring heeft.