Chargebags and Bottles
Secure powder transfer with chargebags and process bottles for aseptic and high-containment pharmaceutical applications. Flexible, compliant and efficient.

Chargebags and Process Bottles – Secure and Efficient Powder Handling
Understanding Containment in
Pharmaceutical Powder Transfer
Handling biopharmaceutical powders requires strict containment measures to maintain sterility, prevent cross-contamination, and ensure operator safety. In pharmaceutical production, containment transfer solutions must meet stringent industry standards while offering flexibility for different applications. Chargebags and process bottles play a crucial role in controlled powder transfer, reducing contamination risks and supporting high-integrity material handling.

Chargebags for Powder ContainmentDesigned for single-use, flexible containment, chargebags provide a cost-effective and disposable solution for handling APIs and other fine powders. Ezi-Dock chargebags come in various configurations. As alternatives, ChargePoint ChargeBag® and GEA Hicoflex® charge bags have set industry benchmarks. Their lightweight design simplifies handling while ensuring maximum protection for both operators and products, making them ideal for high-containment applications that require minimal cross-contamination risks. These bags integrate seamlessly with Ezi-Dock CSV systems and offer Tri-Clamp options, ensuring compatibility with different systems. |
 |
HDPE Process Bottles for Secure Storage and TransferHigh-Density Polyethylene (HDPE) process bottles provide a robust and cost-effective solution for pharmaceutical powder storage and transfer. Ezi-Dock offers its own unique chargebag alternative alongside industry-standard solutions like ChargePoint PuroVaso® plastic containers. HDPE bottles are well-suited for aseptic powder handling in biopharmaceutical environments. Their durable, chemically resistant design ensures safe containment while minimising material waste. These bottles offer environmental protection, ease of handling, and seamless transport, making them particularly valuable in aseptic environments where sterility and containment integrity are paramount. |
 |
Stainless Steel Bottles for High-Integrity ApplicationsFor long-term containment and high-integrity applications, stainless steel bottles offer superior durability and excellent resistance to corrosion and contamination. These bottles are commonly used in high-potency drug production, where robust containment and reusability are necessary. They provide a sustainable and reliable method for powder transfer and storage in pharmaceutical environments where single-use options are not practical. Ezi-Dock offers a range of stainless steel bottles tailored for such demanding applications. |
 |
Ezi-Dock Powder Handling Solutions Overview

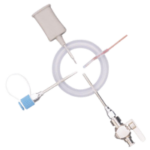
EZI-DOCK – ChargeBag with Tri Clamp Connector

EZI-DOCK – ChargeBag with CSV Connector
Single-use ChargeBag designed for direct connection to EZI-Flow CSV systems, ensuring safe, contained transfer in processes.

EZI-DOCK - HDPE Process Bottles
Durable HDPE process bottles offering secure and efficient containment for powder handling in pharmaceutical settings.

EZI-DOCK – Stainless Steel Process Bottles
Robust, reusable vessels for secure powder storage and controlled handling in pharmaceutical processes.
Manual Filling Processes for Chargebags and Bottles
For pharmaceutical facilities using manual powder handling processes, chargebags and process bottles provide a practical containment solution. Their design ensures compatibility with a variety of powder transfer systems while maintaining high containment integrity. Ezi-Dock offers a range of key components to simplify manual filling, ensuring efficiency and ease of use.
Tools and Accessories for a Complete System
To maximise performance, Ezi-Dock CSV systems can be integrated with additional components, supporting pharmaceutical manufacturing processes and ensuring containment integrity. Customisation options are also available, allowing pharmaceutical manufacturers to tailor solutions to meet specific project requirements and address unique process challenges.

 Single-Use
Single-Use
 Containments
Containments Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Valves
Valves
 Seals
Seals
 Hoses
Hoses
 Fittings
Fittings
 Tube Sealers & Welders
Tube Sealers & Welders
 Transfer Ports
Transfer Ports
 Validation
Validation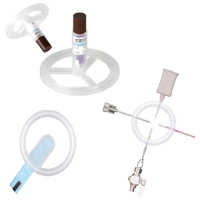







 We utilize cookies to ensure you have the best possible experience while visiting our website.
We utilize cookies to ensure you have the best possible experience while visiting our website.