Containment Transfer Systems
Efficient and contained powder transfer for pharmaceutical production with Ezi-Flow™ CSV systems that prioritise operator safety, sterility and process integration.

High-Containment Powder Transfer Solutions in Pharmaceutical Production
A New Approach to Operator Protection and Efficient Powder Transfer
Handling potent powders in pharmaceutical production demands high-containment solutions to ensure full operator protection, minimise exposure risks, and prevent contamination. Ezi-Dock introduces an alternative approach with its Ezi-Flow™ CSV systems, offering an innovative containment transfer solution focused on safety, efficiency, and seamless integration into existing processes. These systems provide a fully enclosed transfer method, enhancing productivity while reducing reliance on personal protective equipment (PPE) and secondary containment barriers, addressing common challenges in powder handling.
Understanding Ezi-Flow™ CSV Systems
Containment transfer systems play a critical role in pharmaceutical manufacturing, enabling the safe transfer of active pharmaceutical ingredients (APIs) and other high-potency compounds. Traditional powder transfer solutions, such as Containment Split Butterfly Valves from ChargePoint or Andocksysteme Butterfly Valves, have long been industry standards. However, Ezi-Flow™ CSV systems present a lightweight and cost-effective alternative, optimising powder flow, ensuring containment, and maintaining regulatory compliance for pharmaceutical applications.
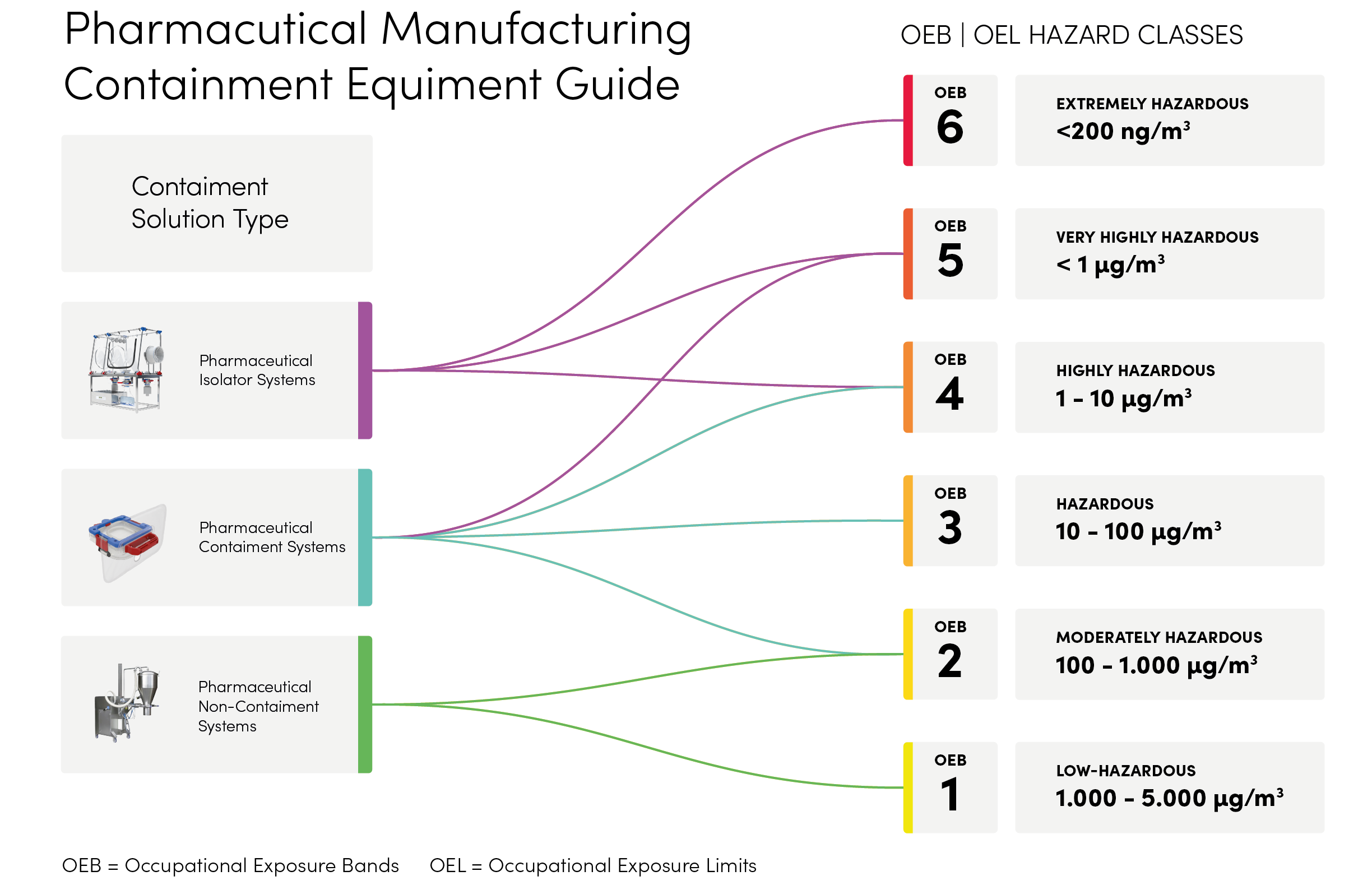
Understanding Containment in Pharmaceutical Powder Transfer
Pharmaceutical containment requirements are defined by Occupational Exposure Bands (OEB) and Occupational Exposure Limits (OEL), ranging from low-hazard to extremely hazardous materials. Selecting the right technology—isolators, high-containment transfer systems, or single-use closed devices—ensures operator safety, product integrity, and compliance with GMP, FDA, and EMA standards.
Romynox provides solutions across all OEB levels, helping manufacturers control potent powders, reduce cross-contamination risk, and streamline validated processes.
Key Benefits of Ezi-Flow™ CSV Systems
Enhanced Operator Safety
Provides complete containment of potent powders, significantly reducing operator exposure to hazardous substances. With an OEB 5 rating and exposure levels of <1µg/m³, it ensures minimal airborne contamination, maximising operator safety.posure and the reliance on PPE or secondary containment barriers.
Compact and User-Friendly Design
Lightweight and space-efficient, allowing for straightforward installation without the need for extensive modifications or additional containment infrastructure.
Material Loss and Yield Optimisation
Enhances powder flow efficiency, reducing material loss and ensuring higher yields in pharmaceutical processes.
Sterility and Cleanability
Designed to meet stringent pharmaceutical hygiene requirements, supporting sterilisation and minimising contamination risks in critical applications.
Seamless Process Integration
Designed for easy implementation within existing pharmaceutical workflows, ensuring process continuity and regulatory compliance.
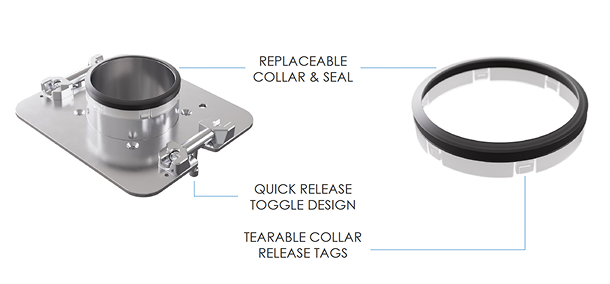
The New Improved
Ezi-Dock CSV MK5
Excel in operator safety
Handling biopharmaceutical powders requires strict containment measures to maintain sterility, prevent cross-contamination, and ensure operator safety. In pharmaceutical production, containment transfer solutions must meet stringent industry standards while offering flexibility for different applications. Chargebags and process bottles play a crucial role in controlled powder transfer, reducing contamination risks and supporting high-integrity material handling.

 |
Enhanced CSV Active UnitDesigned for single-use, flexible containment, chargebags provide a cost-effective and disposable solution for handling APIs and other fine powders. Ezi-Dock chargebags come in various configurations. As alternatives, ChargePoint ChargeBag® and GEA Hicoflex® charge bags have set industry benchmarks. Their lightweight design simplifies handling while ensuring maximum protection for both operators and products, making them ideal for high-containment applications that require minimal cross-contamination risks. These bags integrate seamlessly with Ezi-Dock CSV systems and offer Tri-Clamp options, ensuring compatibility with different systems. |
Upgraded MK5 SpigotHigh-Density Polyethylene (HDPE) process bottles provide a robust and cost-effective solution for pharmaceutical powder storage and transfer. Ezi-Dock offers its own unique chargebag alternative alongside industry-standard solutions like ChargePoint PuroVaso® plastic containers. HDPE bottles are well-suited for aseptic powder handling in biopharmaceutical environments. Their durable, chemically resistant design ensures safe containment while minimising material waste. These bottles offer environmental protection, ease of handling, and seamless transport, making them particularly valuable in aseptic environments where sterility and containment integrity are paramount. |
 |
 |
Enhanced CSV Active UnitDesigned for single-use, flexible containment, chargebags provide a cost-effective and disposable solution for handling APIs and other fine powders. Ezi-Dock chargebags come in various configurations. As alternatives, ChargePoint ChargeBag® and GEA Hicoflex® charge bags have set industry benchmarks. Their lightweight design simplifies handling while ensuring maximum protection for both operators and products, making them ideal for high-containment applications that require minimal cross-contamination risks. These bags integrate seamlessly with Ezi-Dock CSV systems and offer Tri-Clamp options, ensuring compatibility with different systems. |
Ezi-Dock Powder
Handling Solutions
Overview
Ezi-Dock offers an extensive range of powder handling solutions, including chargebags and bottles, designed to meet diverse containment needs in pharmaceutical production. Each component supports safe and efficient powder transfer processes while maintaining compliance with industry standards. Designed for single-use, flexible containment, chargebags provide a cost-effective and disposable solution for handling APIs and other fine powders. Ezi-Dock chargebags come in various configurations.

EZI-DOCK EZI-FLOW™ CSV MK5
Designed for high-containment powder transfer, ensuring that materials remain fully enclosed throughout the transfer process. This system significantly reduces exposure risks and is ideal for pharmaceutical environments demanding strict containment standards.

EZI-DOCK EZI-FLOW™ CSV MK5 All Plastic
Fully plastic version of the MK5, designed for environments requiring single-use solutions. Offers secure, enclosed powder transfer while eliminating cleaning validation, making it ideal for pharmaceutical applications with strict hygiene protocols.

EZI-DOCK EZI-FLOW™ CSV MK5 VHP
Incorporating Vaporised Hydrogen Peroxide (VHP) compatibility, this variant is suited for environments requiring additional sterility controls. It is particularly beneficial in applications where microbial contamination prevention is critical.

EZI-DOCK EZI-FLOW™ CSV MK4
Designed for high-containment powder transfer, ensuring that materials remain fully enclosed throughout the transfer process. This system significantly reduces exposure risks and is ideal for pharmaceutical environments demanding strict containment standards.

EZI-DOCK EZI-FLOW™ CSV MK4 All Plastic
Fully plastic version of the CSV MK4, designed for environments requiring single-use solutions. Offers secure, enclosed powder transfer while eliminating cleaning validation, making it ideal for pharmaceutical applications with strict hygiene protocols.

 Single-Use
Single-Use
 Containments
Containments Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Valves
Valves
 Seals
Seals
 Hoses
Hoses
 Fittings
Fittings
 Tube Sealers & Welders
Tube Sealers & Welders
 Transfer Ports
Transfer Ports
 Validation
Validation


 We utilize cookies to ensure you have the best possible experience while visiting our website.
We utilize cookies to ensure you have the best possible experience while visiting our website.