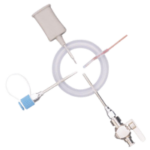
EZI-DOCK - ChargeBag with Ezi-Flow™ CSV Connector
Secure and Efficient Powder Transfer for High-Containment Applications
powder handling in pharmaceutical and biopharmaceutical production. Designed for secure transfer of APIs, excipients, and other sensitive materials, these single-use flexible containers minimise contamination risks and enhance process efficiency. The robust 2-ply polyethylene construction prevents pinholing and ensures durability, even with heavy passive connectors. A non-migrating anti-static additive enhances safety when handling electrostatically sensitive powders. Fully compatible with Ezi-Dock high-containment transfer systems, the CSV connection enables secure, seamless powder transfer while maintaining GMP and cGMP compliance.



Aseptic Powder Transfer | Ideal for sterile manufacturing environments, ensuring contamination-free transfer of excipients, buffers, and active pharmaceutical ingredients (APIs). |
High-Potency API (HPAPI) Handling | Designed for secure containment of highly potent or toxic compounds, protecting both operators and the environment. |
Biopharmaceutical Production | Used in upstream and downstream processing to transfer raw materials, intermediates, and final products while maintaining sterility. |
Sterile API Packaging | Enables safe storage and transfer of APIs and intermediates in controlled cleanroom conditions. |
Closed-System Material Transfer | Minimises cross-contamination risks in cleanrooms, reducing the need for open handling of powders. |
Customised Containment Solutions | Compatible with isolators, glove boxes, and other containment systems for highly controlled environments. |
- High-Level ContainmentEnsures secure, enclosed transfer of potent powders, protecting operators and products.
- CSV ConnectionEnsures secure integration with Ezi-Dock systems.
- GMP & cGMP ComplianceComplies with stringent pharmaceutical production regulations, including GMP, cGMP, and USP standards.
- Aseptic Powder HandlingReduces contamination risks for critical applications.
- Durable & FlexibleSupports high-integrity powder transfer with minimal material loss, reducing waste and simplifying handling.
- Material: Pharma-grade LDPE, 2-ply construction with anti-static properties
- Connection: CSV high-containment valve system
- Containment Level: <1 µg/m³ (OEB 4 and 5 capable)
- Integrity: High-strength, pinhole-resistant design
- Film Properties: Gamma-sterilisable, high-barrier, solvent-resistant
- Compliance: GMP, cGMP, USP Class VI, and FDA 21 CFR 177.1520
- Volume: Available in 5L to 50L configurations
Comparison with Industry Standard Chargebags
Ezi-Dock Chargebags offer a practical alternative to Chargepoint ChargeBag® and GEA Hicoflex® Chargebags, ensuring high-level containment without the complexity of proprietary systems. With their advanced sealing technology and robust material construction, they deliver high containment performance while remaining cost-effective and adaptable to various manufacturing processes.

Question or want to request a qoute?
Feel free to contact us.
Interested in improving your powder containment and handling with Ezi-Dock - Ezi-Flow™ CSV Ready Chargebags? Request a free, no-obligation quote, ask questions, or schedule a demo. Feel free to get in touch with us!
Phone: +31 85 043 3110
Email: info@romynox.nl

 Single-Use
Single-Use
 Containments
Containments Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Valves
Valves
 Seals
Seals
 Hoses
Hoses
 Fittings
Fittings
 Tube Sealers & Welders
Tube Sealers & Welders
 Transfer Ports
Transfer Ports
 Validation
Validation


 We utilize cookies to ensure you have the best possible experience while visiting our website.
We utilize cookies to ensure you have the best possible experience while visiting our website.