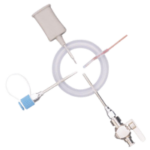
EZI-DOCK - ChargeBag with Ezi-Flow™ CSV Connector
Secure and Efficient Powder Transfer for High-Containment Applications
powder handling in pharmaceutical and biopharmaceutical production. Designed for secure transfer of APIs, excipients, and other sensitive materials, these single-use flexible containers minimise contamination risks and enhance process efficiency. The robust 2-ply polyethylene construction prevents pinholing and ensures durability, even with heavy passive connectors. A non-migrating anti-static additive enhances safety when handling electrostatically sensitive powders. Fully compatible with Ezi-Dock high-containment transfer systems, the CSV connection enables secure, seamless powder transfer while maintaining GMP and cGMP compliance.



|
Aseptic Powder Transfer
|
Ideal for sterile manufacturing environments, ensuring contamination-free transfer of excipients, buffers, and active pharmaceutical ingredients (APIs).
|
|
High-Potency API (HPAPI) Handling
|
Designed for secure containment of highly potent or toxic compounds, protecting both operators and the environment.
|
|
Biopharmaceutical Production
|
Used in upstream and downstream processing to transfer raw materials, intermediates, and final products while maintaining sterility.
|
|
Sterile API Packaging
|
Enables safe storage and transfer of APIs and intermediates in controlled cleanroom conditions.
|
|
Closed-System Material Transfer
|
Minimises cross-contamination risks in cleanrooms, reducing the need for open handling of powders.
|
|
Customised Containment Solutions
|
Compatible with isolators, glove boxes, and other containment systems for highly controlled environments.
|
- High-Level Containment
Ensures secure, enclosed transfer of potent powders, protecting operators and products. - CSV Connection
Ensures secure integration with Ezi-Dock systems. - GMP & cGMP Compliance
Complies with stringent pharmaceutical production regulations, including GMP, cGMP, and USP standards. - Aseptic Powder Handling
Reduces contamination risks for critical applications. - Durable & Flexible
Supports high-integrity powder transfer with minimal material loss, reducing waste and simplifying handling.
- Material: Pharma-grade LDPE, 2-ply construction with anti-static properties
- Connection: CSV high-containment valve system
- Containment Level: <1 µg/m³ (OEB 4 and 5 capable)
- Integrity: High-strength, pinhole-resistant design
- Film Properties: Gamma-sterilisable, high-barrier, solvent-resistant
- Compliance: GMP, cGMP, USP Class VI, and FDA 21 CFR 177.1520
- Volume: Available in 5L to 50L configurations
Ezi-Dock Ezi-Flow CSV MK4 Chargebag Discharging Manual
Ezi-Dock Ezi-Flow CSV MK4 Chargebag filling Manual
Ezi-Dock Ezi-Flow CSV MK4 Double Headed Chargebag Filling Manual
Ezi-Dock Ezi-Flow CSV MK4 Isolator Chargebag Filling Manual
Ezi-Dock PharmaPure AS Range Brochure
Comparison with Industry Standard Chargebags
Ezi-Dock Chargebags offer a practical alternative to Chargepoint ChargeBag® and GEA Hicoflex® Chargebags, ensuring high-level containment without the complexity of proprietary systems. With their advanced sealing technology and robust material construction, they deliver high containment performance while remaining cost-effective and adaptable to various manufacturing processes.

Question or want to request a qoute?
Feel free to contact us.
Interested in improving your powder containment and handling with Ezi-Dock - Ezi-Flow™ CSV Ready Chargebags? Request a free, no-obligation quote, ask questions, or schedule a demo. Feel free to get in touch with us!
Phone: +31 85 043 3110
Email: info@romynox.nl

 Single-Use
Single-Use
 Containments
Containments Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Afsluiters
Afsluiters
 Afdichting
Afdichting
 Slangen
Slangen
 Fittingen
Fittingen
 Tube Sealers & Welders
Tube Sealers & Welders
 Transferports
Transferports
 Validatie
Validatie


 Wij gebruiken cookies om ervoor te zorgen dat u een optimale website-ervaring heeft.
Wij gebruiken cookies om ervoor te zorgen dat u een optimale website-ervaring heeft.