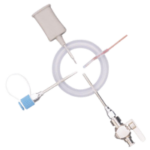
NORDSON MEDICAL – BioEndCap
End Caps with pull tab
Benefits
- Produced from DMF listed polypropylene
- compliant with FDA and USP
- permanently moulded with the production lot number and size identification
BioEndCaps are designed to terminate a disposable manifold until an in-process new sterile connection is to be made.
Produced from DMF listed polypropylene compliant with FDA and USP Class VI and European
Pharmacopeia. Every end cap is permanently molded with the production lot number and size identification. Uniquely constructed pull-tab ensures easy removal of the cap. Suitable for sterilisation by autoclave and gamma irradiation, gamma stable, up to 40 kGy.
Read more about Brand: NORDSON MEDICAL
| Sizes | 1/2″ to 1,5″ |
| Temp. | can be autoclaved at 135°C for 30 min. |
| Connections | Tri-Clamp |
| Material | Natural PP |
| Certificates | FDA, USP Class VI |
No Datasheet PDF files uploaded for this set.
Standard certificates
Optional certificates
You can contact us or ask for your Optional Certificate in your quotation.
No brochures are available at the moment.

 Single-Use
Single-Use
 Containments
Containments Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Valves
Valves
 Seals
Seals
 Hoses
Hoses
 Fittings
Fittings
 Tube Sealers & Welders
Tube Sealers & Welders
 Transfer Ports
Transfer Ports
 Validation
Validation






 We utilize cookies to ensure you have the best possible experience while visiting our website.
We utilize cookies to ensure you have the best possible experience while visiting our website.