3D Single-use bags
Available in 50L, 100L, 200L, 250L, 500L, 1000L, 2000L, 3000L
Benefits
- Customizable to various processes
- Low extractable and precipitation
- Gamma-sterilizable
Is applicable throughout all process steps in biopharmaceutical production.
An alternative to bioprocess bags for storage, sample collection, transfer, and shipping of liquids in all process steps.
Depending on your production scale, we supply the right size bag for an optimal process.
The 3D Bags From COBETTER in: 50L, 100L, 200L, 250L, 500L, 1000L, 2000L, 3000L.
In cases where your production requires other volumes or dimensions, ROMYNOX can provide you with a bag that is tailored to your needs.
Even for small orders, ROMYNOX can assist you. Depending on your needs, we can supply the 3D Bags in different volumes, tubings, connectors and assemblies. The scalability of the 3D Bags allows you to use them throughout the entire biopharmaceutical manufacturing process. Usable from upstream to downstream production (e.g. media and buffer storage, transfer after filtration, storage of intermediates and storage of stock solutions).
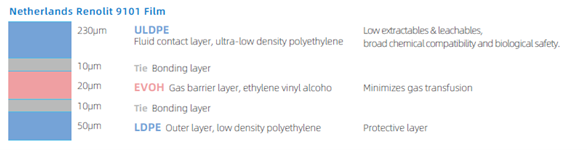
RENOLIT 9101
Renolit 9101 is made up of five layers. ULDPE and EVOH prevent the ingress or egress of oxygen, carbon oxide and moisture. LDPE and HDPE guarantee high process reliability. A visual overview of the composition layers is illustrated below.
RENOLIT 9101
Renolit 9101 is made up of five layers. ULDPE and EVOH prevent the ingress or egress of oxygen, carbon oxide and moisture. LDPE and HDPE guarantee high process reliability. A visual overview of the composition layers is illustrated below.

The 3D single-use bags minimize the risk of cross-contamination and significantly increase safety. Cleaning and sterilization is not required and production efficiency is improved. In addition, it reduces the cost of QC and cleaning verification.
SPECIFICATIONS
- ISO® 9001:2015 quality management system
- ISO class 7 clean zones
- 100% leak test
- ADCF raw materials
- Meet FDA requirements for indirect food additives listed in 21 CFR 177–182
- Meet the requirement of USP <87> In Vitro Biological Reactivity Test
- Meet the criteria of the USP <88> Biological Reactivity Test for Class VI Plastics
- Aqueous extraction contains < 0.25 EU/ml as determined by Limulus Amebocyte Lysate (LAL), USP <85>
- Particles in product eluent meet requirements in USP <788> for large volume parenteral drugs
- Check gamma radiation dose according to ISO® 11137
- Provide a product validation guide and a quality certificate
Read more about Brand: COBETTER
No brochures are available at the moment.

 Single-Use
Single-Use
 Isolators
Isolators
 Mixers
Mixers
 Pumps & Flow Measurment
Pumps & Flow Measurment
 Valves
Valves
 Seals
Seals
 Hoses
Hoses
 Fittings
Fittings
 Tube Sealers & Welders
Tube Sealers & Welders
 Transfer Ports
Transfer Ports
 Validation
Validation





 We utilize cookies to ensure you have the best possible experience while visiting our website.
We utilize cookies to ensure you have the best possible experience while visiting our website.